An interesting concept I’ve stumbled across during my reading into neurobiology is that a neurotransmitter need not be exclusively excitatory or inhibitory. In fact, a prime example of this would be glutamate in phototransduction, the process by which the stimulation of photoreceptors by photons triggers the propagation of an action potential across the optic nerve.
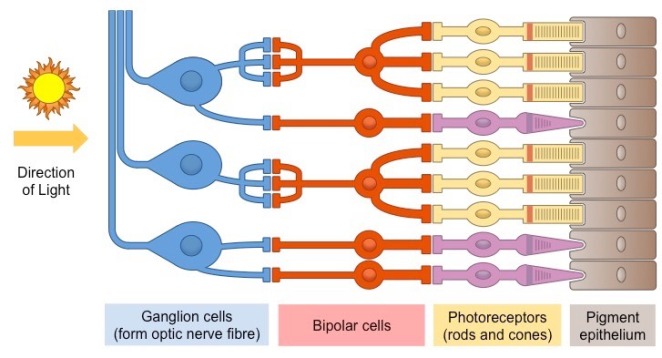
A pretty good minimalist diagram from Bioninja that shows the basic structure of the retina
The behaviour of photoreceptors in the dark
In the dark, we find that the photoreceptor cells have a relatively high membrane potential (-40mV). This membrane potential comes about because, in the dark, these cells have high concentrations of a molecule called cyclic guanosine monophosphate (cGMP), which binds to cGMP gated Na+ and Ca2+ channel proteins to allow their entry by facilitated diffusion into the photoreceptor, hence the cell to exhibit a depolarised membrane potential.
This has the effect of keeping voltage gated Ca2+ channels between the synaptic knob and the main body of the photoreceptors open, thus facilitating the movement of glutamate across the synaptic cleft and allowing it to bind to receptors on the post-synaptic bipolar cell.
This is where things get interesting. Glutamate is typically a fast-acting, excitatory neurotransmitter, but in this particular case, it behaves as a slow-acting inhibitory neurotransmitter. I initially thought it was able to do this by binding to a glutamate gated anion channel.
As it would seem, glutamate activates a cytoplasmic guanosine (G) protein by binding to receptors on the membrane of the bipolar neuron. This G proteins initiates a cascade of reactions that reduce the cytoplasmic cGMP concentration, thus reducing binding of cGMP to cGMP gated cation channels that need to be open to trigger depolarisation.
As a result, in the dark, the bipolar neuron is hyperpolarised (its membrane potential is approximately -65mV) due to the glutamate released by the depolarised photoreceptors. The bipolar cell’s inability to depolarise is what inhibits any impulses from being transmitted from the eye to the brain under pitch-black conditions.
What is fascinating is how the membrane potential across the bipolar cell changes when light strikes the photoreceptor beneath it.
The behaviour of photoreceptors under illumination
In regions called receptor disks, photopigments are stored within photoreceptor cells. In rods, the main photopigment is rhodopsin – a molecule associated with retinal and a number of opsin polypeptides. When retinal absorbs a photon, its configuration changes from its 11-cis isomer to an all trans isomer. This initiates a series of reactions which result in the activation of the intracellular signalling protein transducin (from which phototransduction derives its name).
Transducin in turns activates phosphodiesterases which hydrolyse cGMP molecules. This has the effect of reducing the concentration of cGMP in the photoreceptors, thus reducing binding to cGMP gated Na+ and Ca2+ channel proteins, thus resulting in hyperpolarisation of the photoreceptor cell.
This decrease in membrane potential closes the voltage gated Ca2+ channels, reducing the concentration of Ca2+ in the synaptic knob. As a result, the quantity of glutamate released sharply decreases, leading to the depolarisation of the bipolar cell, triggering the propagation of an action potential along the axons of the ganglia.
Moving away from neurotransmitters momentarily, let’s look into how cytoplasmic Ca2+ concentrations can influence the extent to which photoreceptors respond to light.
Sensitivity to Light Intensity
Generally, photoreceptors display diminishing sensitivity to illumination as light intensity increases. When the Ca2+ concentration in the photoreceptor decreases, it triggers a small change in the phototransduction cascade, by initiating the upregulation of the enzyme that produces cGMP and also increasing the affinity the cGMP gated Na+ and Ca2+ channels have for cGMP. This reduces the sensitivity of the photoreceptors to any increases in illumination by requiring a larger number of activated photopigments to reduce the cytoplasmic cGMP concentration to a low enough level such that the membrane potential can be decreased.
Recycling of retinal
With respect to the photopigments, there must always be a sufficient quantity of viable photopigments in the receptor disk. To ensure that this is the case, following the depolarisation of the photoreceptor, the protein arrestin binds to activated rhodopsin molecules, preventing them from binding to transducin and facilitating their movement to the pigment epithelium.
In the pigment epithelium, a series of enzymatic reactions convert the all trans retinal molecule in rhodopsin into its 11-cis form. Following this, the rhodopsin molecule moves back into the receptor disk. This system effectively allows for the recycling of previously activated photopigments and maintains the presence of inactivated photopigments in the receptor disk.
Conclusion
Phototransduction is an example of a process whose beauty lies in the rationality of its design – it continuously regenerates new components, gives rise to variable light sensitivity, and most importantly, allows us to see.

Wow, this website is great!
LikeLiked by 1 person
Hey David, I agree! The amount of resources on this website is absolutely amazing.
LikeLiked by 1 person
😂🤣
LikeLike
Although I do not attend IB Biology, I agree that this website is great. 👍🏽
LikeLiked by 1 person
I propose that while Phototransduction is indeed an interesting concept, its’ clinical potential is limited due to its’ restricted nature.
LikeLike